ISO 14971 Risk Management for Medical Devices
IBM ELM supports companies developing medical devices to comply with the ISO 14971 with several assets described below.
Softacus has provided the templates and assets and those will be given for free with the licenses.

About ISO 14971 support
IBM ELM Solution help software engineers identify, analyze, mitigate and reduce risks that might affect the product’s success. It facilitates compliance with the risk-related requirements of ISO 14971 Risk Management for Medical Devices and other safety-critical standards.
They offer comprehensive software engineering risk management features that are fully integrated with its Application Lifecycle Management capabilities:
-
Risk Analysis and Risk Analysis methods (FTA and FMEAs)
- Intended Use Validation
- Configurable KPI Dashboards
- CAPAs
- Customizable Processes and Workflows
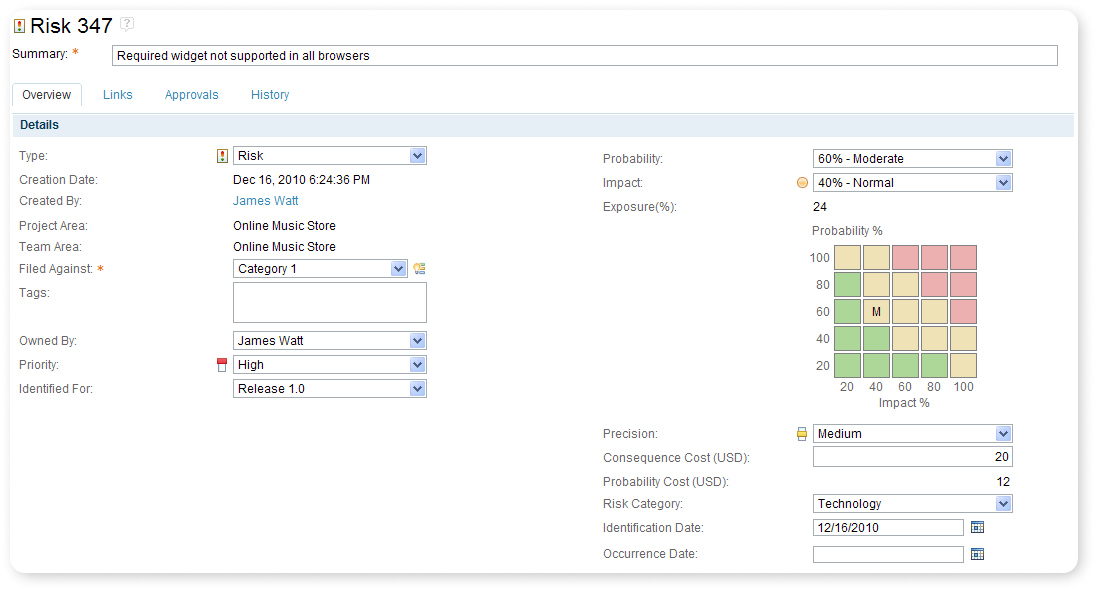
Risk Identification
IBM Application Lifecycle Management offers compliance throughout the whole Design Process with Risk identification by implementing configurable artifacts with the ability to link individual risk requirements, risks, hazards, risks mitigations, and all the attributes your team needs.
Traceable from Start to End. Top-down or bottom-up. It is up to you and your team.
Configurable KPI Dashboards
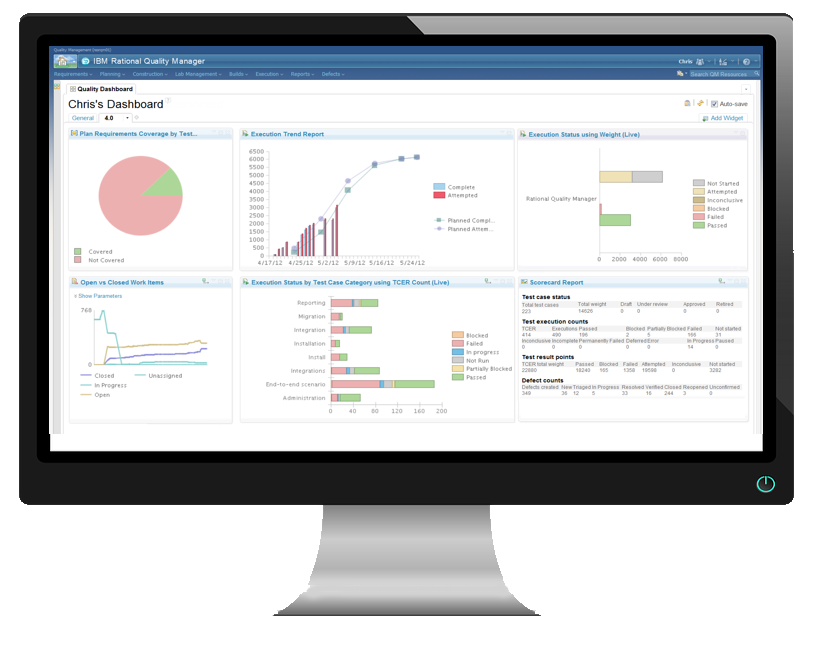
The key performance indicator dashboard consists of reports that keep the user aware of the current performance of projects' progress without requesting further information.
After logging in, the user with KPI Dashboard privileges can view the KPI dashboard and see an overall view on the Dashboard.
IBM ALM provides preconfigured KPI Dashboards for individual Project types that can be tailor-made for our clients' needs.
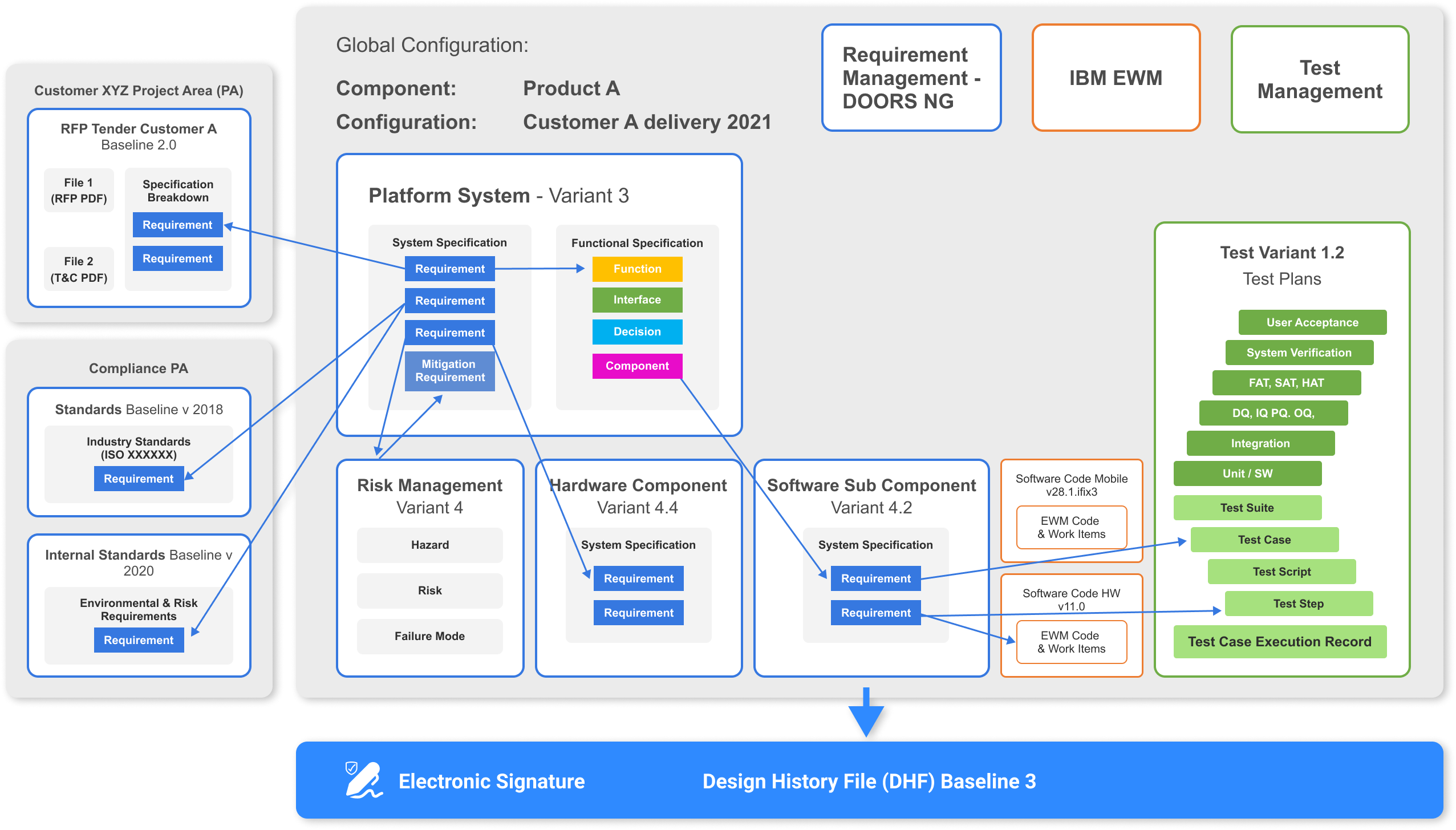
Design History File (DHF)
Achieve single source of truth for all your domain data.
Maintain and generate on single click or on schedule. Track changes and link them to change requests. Take advantage of automated audit trail. Set specific permissions in order to achieve granular rights for specific user groups.
Document , use, adapt and enact medical devices processes quickly. Ensure quality through whole Development Lifecycle by using state of the art IBM Tools.
Medical devices relevant informations
IEC 62304 support
IBM provides out of the box Process Template for IEC 62304 in the area of system and software development for medical devices.
ALM for medical devices
You can get the strongest solution on the market for ALM Engineering. This Solution includes requirements, planning, test management, MBSE.
Strong reuse
Reuse your requirements, source code, tests, models, as well as whole system components. IBM ALM supports branching, merging and global configuration Mangement.
Corrective and Preventive Actions (CAPA)
IBM offers the best solution on the market for the ideal CAPA System for your needs. Our integrated CAPA system offers systematically capturing and investigating complaints, defects, process failures, or deviations.
Integrated CAPA system manages corrective and preventive actions through automated workflows and design processes configurable for our client's needs, while also being able to retrieve important documents for audits at any time.
Our customers have reported that using our integrated CAPA system reduces Problem Resolution times, partly thanks to the simplicity of finding critical data for analysis when you and your team need it.
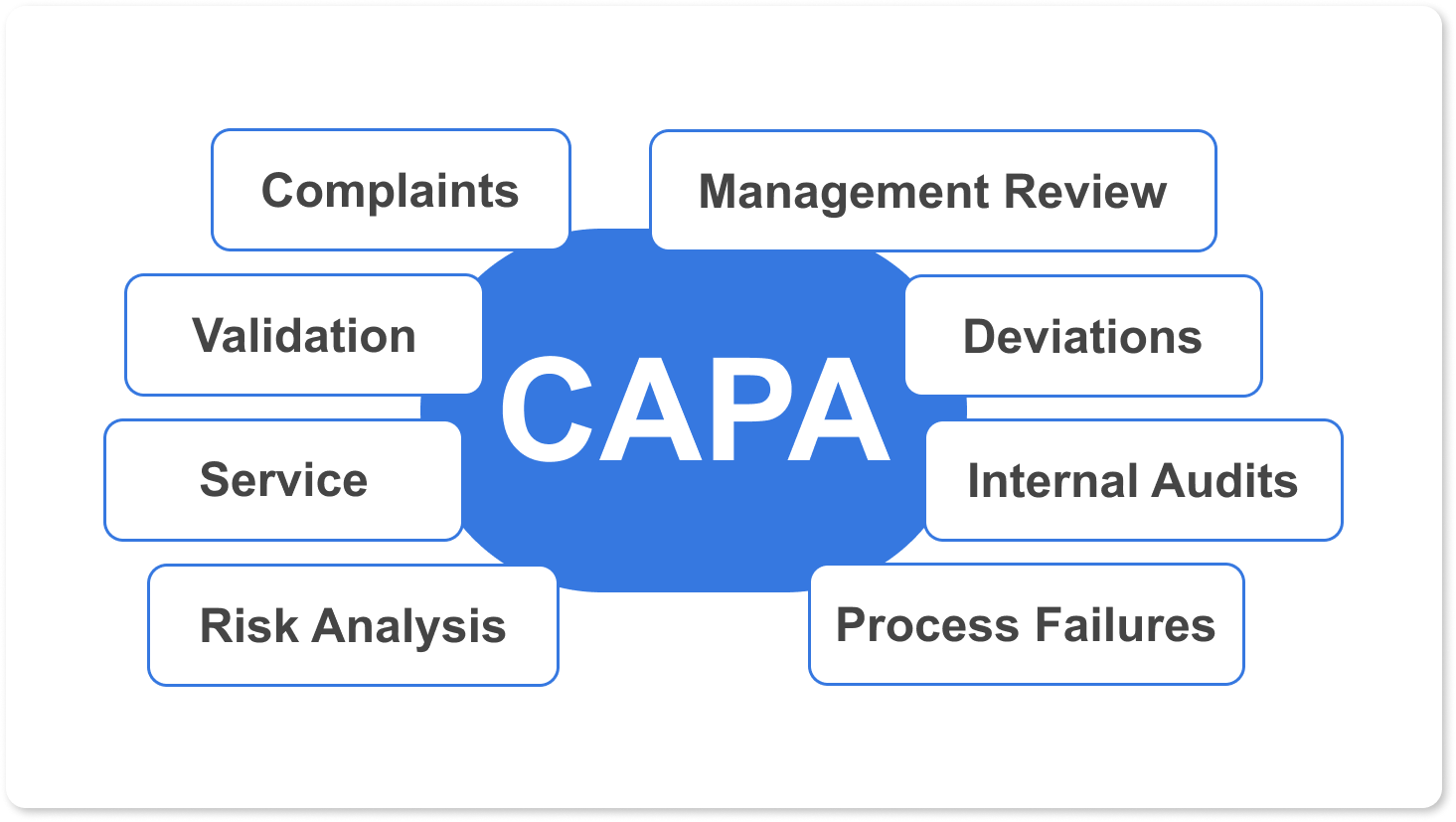
Automated Workflows
Make best use of the IBM Application Lifecycle Management with its fully customizable processes taking advantage of the automated Workflows that helps your teams with collaboration by market-leading Dates and Updates Documentation assuring no unnecessary mistakes like editing or sending out older versions of documents are made during the Design Process.
Intended Use Validation
IBM Application Lifecycle Management offers customizable templates for Intended Use Validation (IUV) in your Risk Management File.
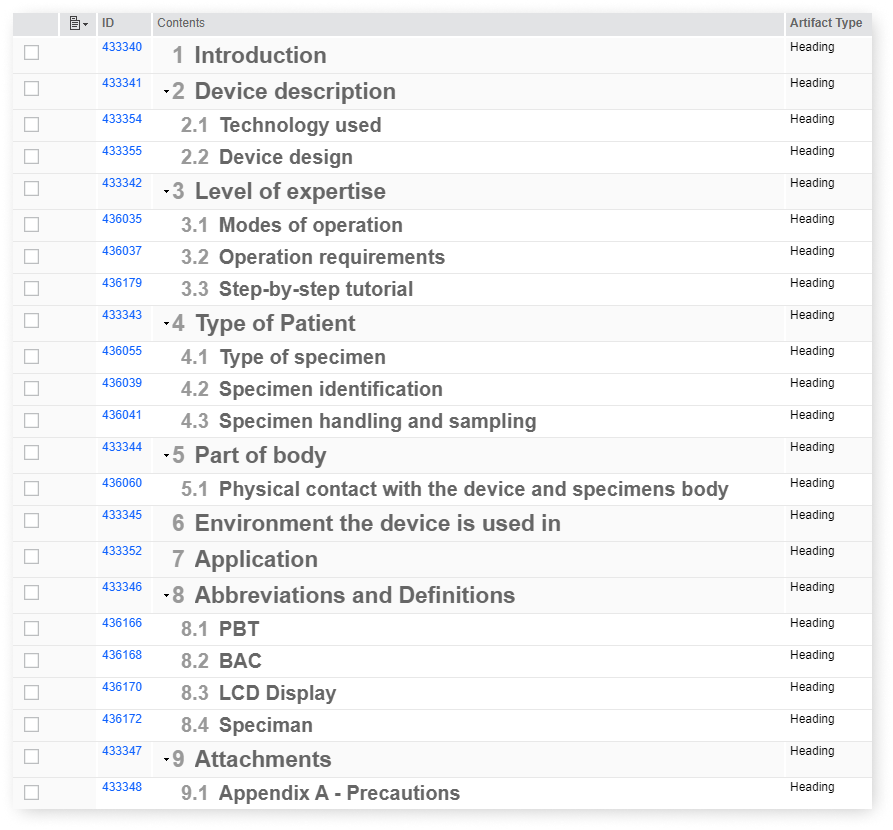
- Include detailed Specifications such as:
- Device description
- Level of expertise required
- Type of Patient and Environment the device will be used in
- important documents regarding Intended Use Validation
- diagrams, infographics and schemes
- manuals or setup guides and many more
All in one Document with the ability to trace all the important information and details while being able to link the specifications to individual requirements they satisfy.
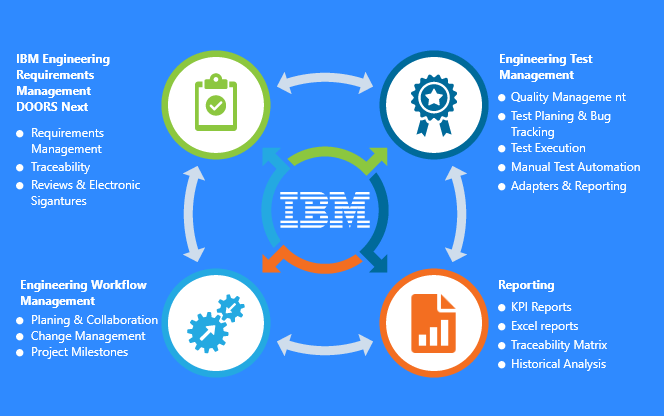
IBM ELM Solution Parts
Requirements Management
Single source of truth for your requirements, specifications and other artifacts. Use traceability to assess impact of a change to ensure compliance with ISO 14971.
Planning
Get full control of work items with features like cross-project planning, work reviews and high-level collaboration while being able to link tasks, stories, change requests…
Software Configuration Management
IBM ELM allows you support to systematically organize, manage, and control the changes in the documents, codes, and other important data sources.
Reporting and Publishing
You can get professional documents on click of a button or delivered to your mailbox on schedule. You can also create nice KPI charts and get them via Dashboard or as "live" Excel.
Model Based System and Software Engineering
Deliver higher quality systems and software faster by managing product and systems development with a solution for collaborative design, development and testing activities.
Test Management
Increase efficiency and quality of systems and software delivery with test planning, workflow control, tracking, and metrics reporting across multiple environments.
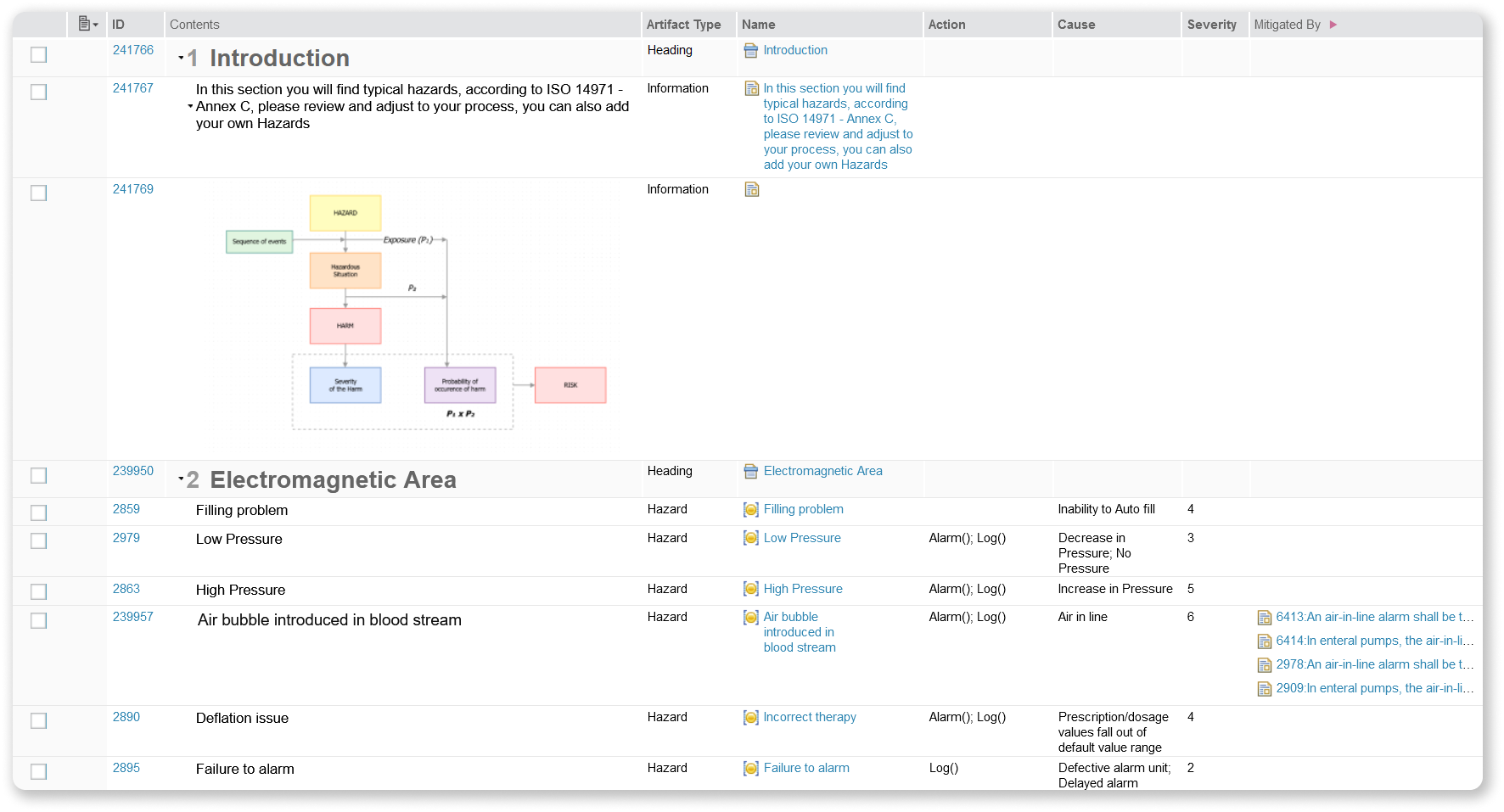
Hazard Types Template
IBM ALM and Softacus provide a pre-defined, yet fully customizable Base list of Hazards, coming directly from ISO 14971 - Annex C. It showcases a few examples of Hazard types, example of Hazards, Cause, Severity and everything else you would need.
We support and develop templates that are given to you for free with the licenses. Contact us here for more info about medical templates and presentations for ISO 14971 Risk Management for Medical Devices
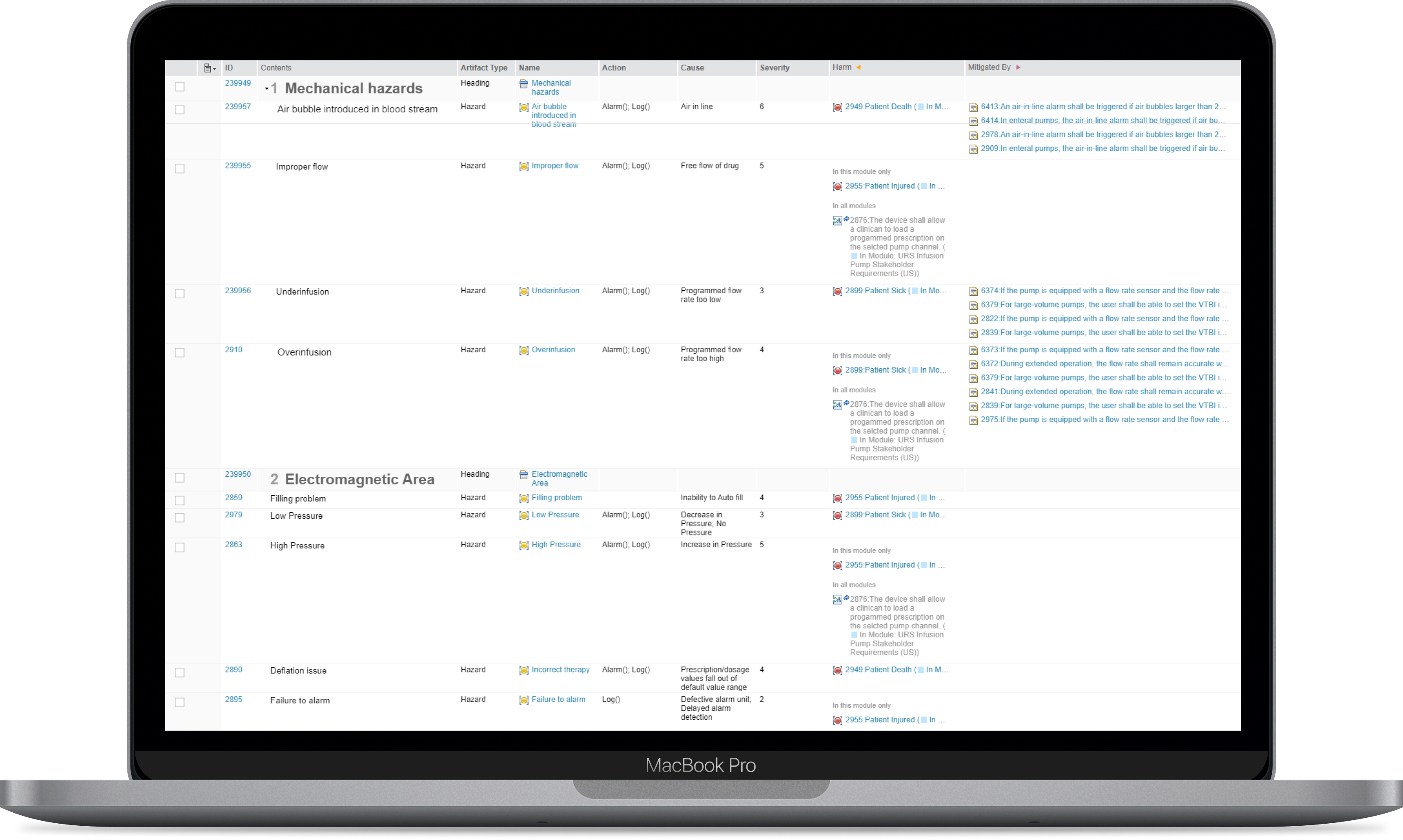
Risk Analysis
IBM Application Lifecycle Management supports Hazard and Risk Analysis (HARA) helps you to identify the intensity of risk of harm to people and property.
IBM ALM provides key advantages that help you throughout your Design Process and satisfy your Design Needs such as:
- Linking artifacts and important elements in your Risk Management File with affected requirements
- Easy-to-use Process Simulations
- Single Source of Truth state of software implementation
- Dates and Updates Documentation
- Implementation of Collaboration among individual teams
Risk Analysis Methods
Failure Mode and Effects Analysis (FMEA)
IBM Rational software provides Failure Mode and Effects Analysis (FMEA) feature set. This enables the product development team to identify, define, and control the mitigation of risks while ensuring traceability between requirements, failure modes, and test cases.
IBM provides out of an box FMEA extension for IBM Jazz Platform which is installed in /RM component. The extension enables users to:
- Choose attributes like probability, severity, impact
- Automated or manual calculation of risk priority number (RPN)
- Linking of FMEA artifacts to affected requirements and mitigation requirements
- Out of the box Gap Analysis
- Compare RPN before and after mitigation
- Color-code RPN based on the value
- Adjust the FMEA calculation to any of your needs
Fault Tree Analysis (FTA)
A deductive (top-down) approach that links causal factors (e.g. faults, conditions, and events) together via logic operators to create hazards
- Traceable links among requirements, design, and safety elements makes your “safety case”
- FTA elements contain metadata:
- Severity
- Likelihood
- MTBF
- Risk
- Safety Integrity Level
- UML Fault Tree Analysis Profile
- Fault Means, Effect, and Criticality Analysis (FMECA)
- Security Analysis Diagram
- UML Profile for modeling cybersecurity, threats, and countermeasures
IBM ELM Platform Components